Novel oxides
We explore a variety of different semiconducting metal oxides. Most of them are sesquioxides with an M2O3 (M = metal) structural formula and occur as polymorphs, i.e. existing in several different crystal structures.
In2O3
Indium oxide is a technologically important material known for high conductivity when doped by Sn while maintaining transparency in the visible spectral range. Its typical applications are therefore found in transparent electronics, plasmonics or as front contacts for optical devices such as solar cells or thin film optical displays. [Feneberg et al., Phys. Rev. B 93, 045203 (2016).]
Here we explore In2O3 in its stable cubic form Bixbyite and the metastable corundum structure (α-In2O3).
Bixbyite In2O3
The stable form of indium oxide is cubic (bixbyite) and has 40 atoms in its unit cell. This leads to interesting properties, like 16 infrared active optical phonons, a variety of valence bands, and complications in first-principles calculations due to the extreme computational cost. We investigated single crystals and epitaxial thin films with different free-electron concentrations to study man-body effects on the band structure, the conduction band nonparabolicity, and effective electron masses.
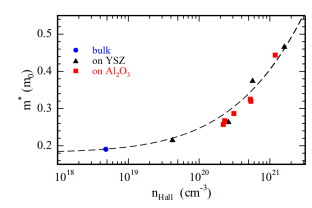
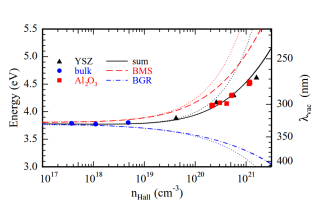
Furthermore, the dielectric function up to a photon energy of 40 eV yielded the possibility to benchmark first-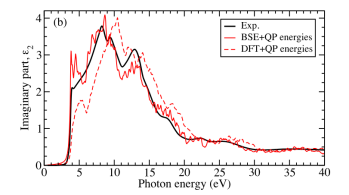 principles calculations and shed light onto the effect of lattice screening.
principles calculations and shed light onto the effect of lattice screening.
Bixbyite InxGa(1-x)2O3
Alloys of In2O3 have been explored as well:
- Feldl et al., Appl. Phys. Lett. 119, 042101 (2021).
- Papadogianni et al., Phys. Rev. Materials 6, 033604 (2022).
α-In2O3
With α-In2O3 bandgap engineering in the α-In2O3–α-Ga2O3–α-Al2O3 system opens the possibility of tuning the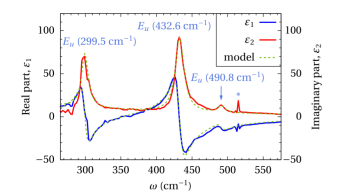 bandgap from 3.8 to 8.8 eV. We invetigate the Raman and IR-active phonon modes along with the absorption behavior at the bandgap.
bandgap from 3.8 to 8.8 eV. We invetigate the Raman and IR-active phonon modes along with the absorption behavior at the bandgap.
Ga2O3 and their alloys
Gallium oxide is a wide band-gap semiconductor suitable for application in high-power electronic devices due to its high break-down voltage. It can crystallize in severall different polymorphs, which have similar basic properties such as a band-gap size of around 5 eV. Monoclinic β-Ga2O3 represents the thermodynamically stable phase. We explore the other less researched phases and their alloys in terms of their optical properties.
[Kracht et al., Phys. Rev. Applied 10, 024047 (2018).]
α-Ga2O3
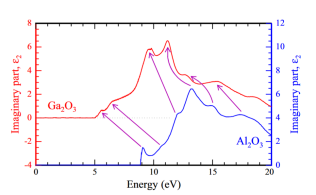
Corundum-like Ga2O3 is a metastable polytype of gallia. Intriguingly, it has the same crystal structure than sapphire (α-Al2O3), is thus alloyable, and it can be donor doped. We are interested in the fundamental optical properties, its comparison to sapphire, the anisotropy, and the effective electron mass.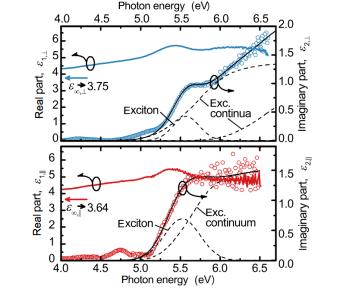
- Segura et al., Phys. Rev. Materials 1, 024604 (2017).
- Kracht et al., Phys. Rev. Applied 10, 024047 (2018).
- Feneberg et al., Phys. Rev. Materials 2, 044601 (2018).
α-(AlxGa1-x)2O3
As Corundum-like Ga2O3 and sapphire (α-Al2O3) share the same crystall structure it is alloyable with Al. We are 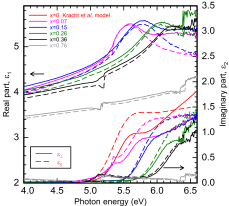 interested in the fundamental optical properties of the alloy. Specifically the anisotropy of the dielctric funtion.
interested in the fundamental optical properties of the alloy. Specifically the anisotropy of the dielctric funtion.
α-(TixGa1-x)2O3
Similar to AlGa2O3 alloing with Ti offers the possibility of badgap 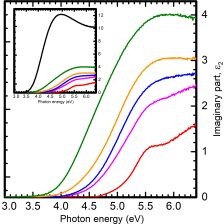 energinering over a wide range. We explore the shift of the absorption onset of only ~20 nm thick films on sapphire subtrate with incresing Ti-content.
energinering over a wide range. We explore the shift of the absorption onset of only ~20 nm thick films on sapphire subtrate with incresing Ti-content.
γ-Ga2O3, κ-Ga2O3 and κ-(InxGa1-x)2O3
 explored cubic γ-phase and the orthorhombic k-phase, whereby specifically the anisotropy arising from the three different lattice parameters is important to understand.
explored cubic γ-phase and the orthorhombic k-phase, whereby specifically the anisotropy arising from the three different lattice parameters is important to understand.
Based on the study of κ-Ga2O3 we exapanded our resereach to κ-(InxGa1-x)2O3 to determine the shift of the different interband transitions with In-content.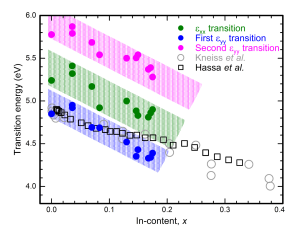
Rutile-GexSn1-xO2
The wide bandgap semiconductor rutile SnO2 when heavily n-doped is well known to be a transparent conducting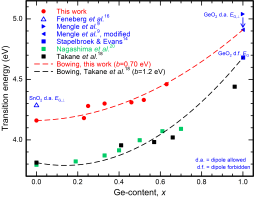 oxide (TCO) and can be used, e.g., in solar cells, displays, or smart windows. Rutile GeO2, on the other hand, is a semiconductor of recent interest, as it is theoretically proven to allow an ambipolar doping and a high carrier mobility. We investigate rutile GexSn1-xO2 thin films on TiO2. An evaluation of the dielectric function yields the characteristic transition energies at the absorption onset, which lead to a bowing parameter of the bandgap.
oxide (TCO) and can be used, e.g., in solar cells, displays, or smart windows. Rutile GeO2, on the other hand, is a semiconductor of recent interest, as it is theoretically proven to allow an ambipolar doping and a high carrier mobility. We investigate rutile GexSn1-xO2 thin films on TiO2. An evaluation of the dielectric function yields the characteristic transition energies at the absorption onset, which lead to a bowing parameter of the bandgap.
ZnGa2O4
The cubic spinel structure of ZnGa2O4 exhibits intrinsic n-type conductivity, caused by GaZn antisites. We explore 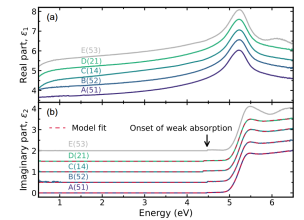 bulk single-crystals by spectroscopic ellipsometry and Raman spectroscopy. By analyzing the contribution of free carriers in the IR- region, experimental values of the electron effective mass are determined. Investigations in the visible to UV region allow the identification of the direct band gap of the inverse spinel structure and refinement of the direct band gap energy of the normal spinel.
bulk single-crystals by spectroscopic ellipsometry and Raman spectroscopy. By analyzing the contribution of free carriers in the IR- region, experimental values of the electron effective mass are determined. Investigations in the visible to UV region allow the identification of the direct band gap of the inverse spinel structure and refinement of the direct band gap energy of the normal spinel.